At the invitation of the editors of Nature Chemistry, scientists from Poznań compared the newly developed method of obtaining optically active compounds with those previously described in the literature. The advantages of the method as well as some shortcomings were indicated, and potential directions for further work were identified ("The gains from breaking symmetry". J. Gajewy and M. Kwit, Nature Chem. 13, 623-624 (2021);
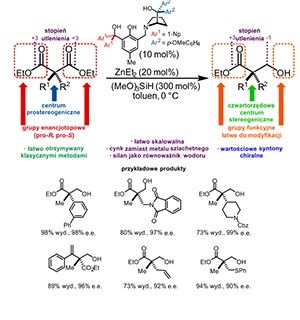
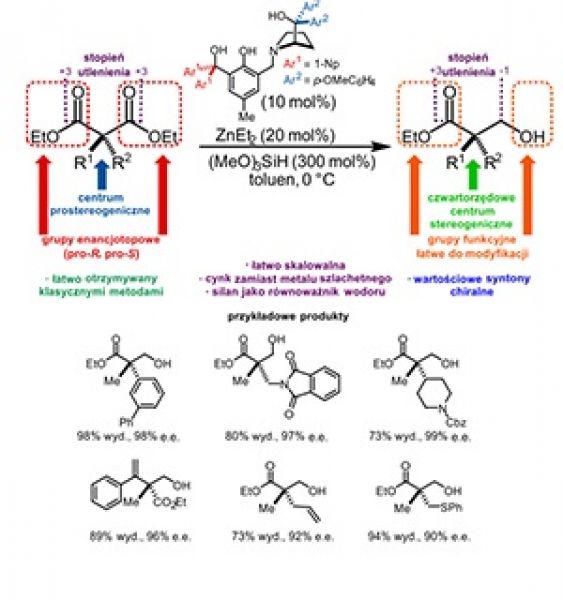
The general method providing easy access to bioactive compounds with a quaternary stereogenic center is a Holly Grail of stereoselective synthesis. This has now been achieved by combining the classical organic transformations with the discovery of a new zinc-based catalytic system for the desymmetrization of prochiral α,α-disubstituted malonic esters.
Nature, although it is said to be an inexhaustible source of chiral products, usually provides compounds in one enantiomeric form, leaving the problem of acquiring the opposite enantiomer open. Therefore, throughout the years there has been observed a shift from methods utilizing raw materials of natural origin towards methods of synthesis using either stoichiometric or catalytic amounts of chirality inducers (chiral auxiliary or catalysts). The latter constitutes currently dominant approach in chemistry. “Right” catalysts used in asymmetric synthesis should be, at the same time, highly active, readily available, and cost-effective. As one can guess, it is usually impossible to meet these requirements at the same time.
Being an alternative to the formation of chirality element(s) and to the resolution of racemates, desymmetrization reactions appear to be an elegant solution to the problem of obtaining optically active compounds in enantiomerically pure form. In desymmetrization reactions, precluding chirality, the symmetry element(s) is (are) lost, thus the prochiral molecule will convert into the chiral one. In the simplest case, for two the same functional groups present in a molecule and attached to the same carbon atom, one undergoes chemical transformation and changed into a different one. This approach may be especially useful in cases where compounds, having quaternary carbon, will constitute the desired target. However, the restricted accessibility of prochiral substrates, the tendency to overreaction providing achiral bis-functionalized products and not easy enantiocontrol preclude desymmetrizations in daily laboratory practice.
Recently developed a new method for desymmetrization of double-substituted malonate derivatives has provided easy access to the diverse array of chiral synthons containing a quaternary stereogenic center in the molecule skeleton and are characterized by great utility in synthesis. In this method, zinc-catalyzed asymmetric hydrosilylation (AHS) has been applied for the reduction of one of the ester groups present in the substrate molecule. In this reaction, hydroslilane constitutes a hydrogen gas surrogate, so hydrosilylation might be consider an addition of silylated nucleophile (hydride) to the carbonyl group.