Maciej Bujak, Marcin Podsiadło and Andrzej Katrusiak, Acta Cryst. (2021). B77, 632-637.
DOI: 10.1107/S2052520621006399
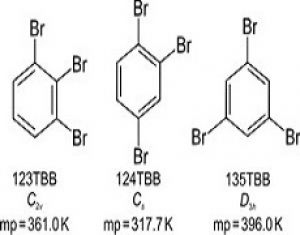
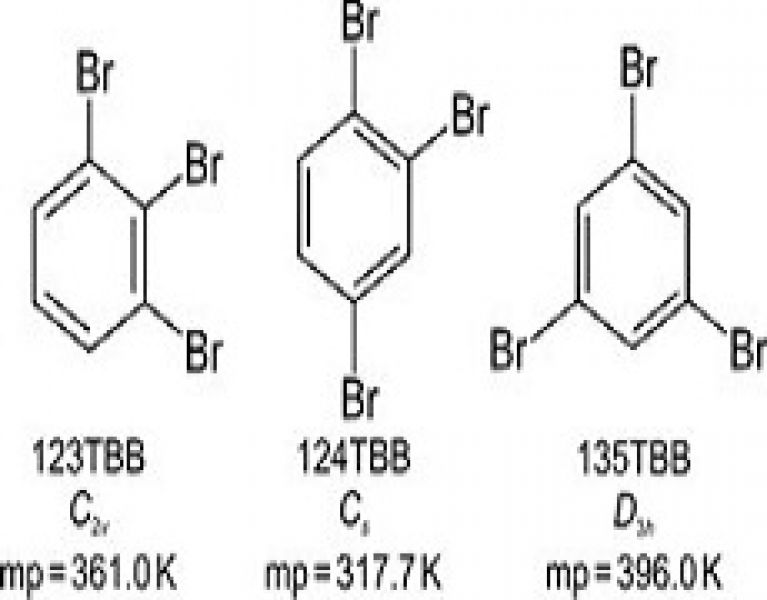
The melting points of tribromobenzene isomers have been correlated with the number, nature and distribution of intermolecular interactions in their structures.
Single crystals of isomeric 1,2,3-tribromobenzene (123TBB), 1,2,4-tribromobenzene (124TBB) and 1,3,5-tribromobenzene (135TBB) have been grown from different solvents and their structures determined by X-ray diffraction at 100, 200 and 270 K. The melting-point differences of ca. 40 K between 135TBB, 123TBB and 124TBB have been correlated with the molecular symmetry and packing preferences in the crystal, as well as with the main types of intermolecular halogen interactions, i.e. Br⋯Br, Br⋯C (Br⋯π) and Br⋯H.
The crystals of three tribromobenzene isomers have been investigated at low temperature in order to explore the relationships between the intermolecular interactions and melting points of these compounds. Beside the symmetry, described by Carnelley’s rule, the intuitive principle for the melting-point differences can be associated with cohesion forces: the stronger and more frequent the interactions between molecules the more energy is required to melt the crystal. The present results show that the strongest halogen bonds alone cannot account for the melting-point differences. Therefore weaker intermolecular interactions have to be considered too. Higher molecular symmetry provides better access to the Br atoms and hence the specific optimum interactions that correlate with the higher melting point of the particular isomer.