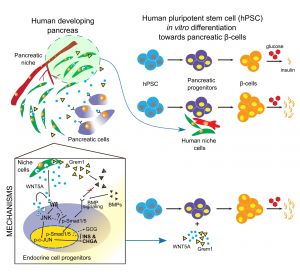
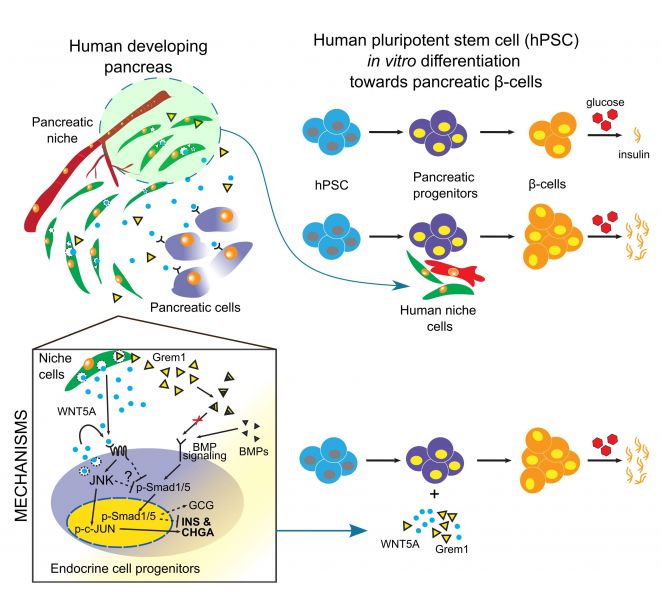
Human pancreatic microenvironment promotes β-cell differentiation via non-canonical WNT5A/JNK and BMP signaling
Jolanta Chmielowiec # 1, Wojciech J Szlachcic # 2, Diane Yang # 1, Marissa A Scavuzzo 3, Katrina Wamble 4, Alejandro Sarrion-Perdigones 5, Omaima M Sabek 6 7, Koen J T Venken 5 8, Malgorzata Borowiak 9 10 11 12 13
Nat Commun. 2022 Apr 12;13(1):1952.
doi: 10.1038/s41467-022-29646-1.
Diabetes is an increasing problem worldwide as the number of patients is rapidly growing. Diabetes is characterized by elevated glucose levels in the blood due to the insufficient amount of insulin, a hormone that enables glucose entry into the peripheral cells and thus glucose clearance from the blood. Without glucose some cells will starve and eventually die like neurons, while in other cell types the glucose deficit will cause significant metabolic changes. Furthermore, the accumulation of glucose in the blood will lead to the systematic damage in basically all organs in the human body. Diabetes is a great target for regenerative medicine as there is only one type of cells that produce insulin in humans, and these are the endocrine beta cells in the pancreas. Therefore, it we can find a new robust source of human beta cells that could be transplanted to the patients, we might find not an improvement but a cure for diabetes.
In this project, we asked a simple, yet critical question of how microenvironment affects development of insulin-producing pancreatic beta cells in the human context. We first established that microenvironment, defined as a combination of mesenchymal and endothelial cells, differs significantly during human pancreatic development. Consequently, only stage-specific microenvironment is beneficial for beta cell formation from human pluripotent stem cells. Next, we asked what is so special, different about these mesenchymal and endothelial cells at this particular stage and using unbiased RNA-sequencing we identified a cohort of secreted stage-specific growth factors and components of extracellular matrix. In parallel efforts, we identified the transcriptomes of human stem cell-derived beta cell progenitors and looked for the pairs of signaling molecules where ligand is produced by mesenchymal and endothelial cells, while the beta cell progenitors express the receptors, thus are equipped to respond to these signals. We then started the most mundane part of the project, where we tested the role of each of such signaling modules during human stem cell differentiation towards beta cells. As result, we identified Wnt5a, a growth factor that specifically enhanced the human beta cell formation in vitro as well as improved their function, meaning the insulin secretion in the response to the increasing glucose concertation. We then performed yet another global transcriptome analysis to uncover what is molecular mechanism behind Wnt5a pro-beta cell activity. This gave us some valuable clues, candidate signaling-transcriptional networks, that we further investigated using the multiple luciferase reporters or imaging flow cytometry, collectively suggesting that Wnt5A first activates c-Jun signaling pathway in the differentiating beta cells and then Wnt5a inhibits the BMP4 signaling. These coordinated events together dramatically increase human beta cell formation and function in vitro.