Kinga Roszak and Andrzej Katrusiak High-pressure and environment effects in selenourea and its labile crystal field around molecules Acta Cryst. (2021). B77, 449-455
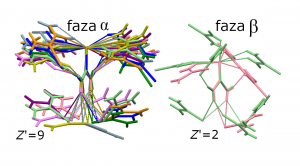
Selenourea, urea derivative, forms crystals of enantiomorphic space-group symmetry, with 9 independent molecules in the structure (Z’). Such structures with Z’=9 are very rare and only 17 of them have been evidenced so far, which constitutes about 1.2·10-5 of crystals currently deposited in the Cambridge Structural Detabase. Selenourea is by far the smallest and the simplest compound in this group of 17 crystals, by the terms of the number of atoms and the rigid structure of the molecule. Thus, the high Z’ in selenourea cannot be due to conformational differences between molecules, but it is caused solely due to their different packing motives.
The study on selenourea were performed to check if high pressure would increase or decrease its Z’ number and what would be the mechanism of such a change.
High-pressure experiments were performed in the Merrill-Basett diamond anvil-cell either by gradually isothermal compressing a single crystal, or high-pressure isochoric recrystallizations and growing single crystals. Methanol and aqueous solutions were used as a pressure transmitting media. The structures of high pressure phases were determined by single crystal diffraction methods. Ambient-pressure, trigonal, noncentrosymmetric phase alpha of selenourea, (Z’=9) under high pressure conditions undergoes several intriguing transformations, depending on the pressure-transmitting medium and the compression or recrystallization process.
In glycerine or oil, selenourea transforms into phase beta at 0.21 GPa, above this pressure the sample crystal started to crack. High-pressure recrystallization of aqueous solution yielded the same α- selenourea up to 0.21 GPa, both at isothermal and isochoric conditions. The single crystals of alpha-selenourea grown of aqueous solution could be compressed to 0.30 GPa, above this pressure it crashed, similarly as in the previous experiments. Above 0.50 GPa selenourea duohydrate was obtained, and in order to prevent its formation for the next crystallizations the methanol solution was used instead of water. These recrystallizations yielded a new centrosymmetric phase beta (Z’=2), it is stable to 3.20 GPa at least. The high-pressure study of alpha-selenourea indicates on the intriguing aspects of its structure, such as the high Z’, the enantiomeric symmetry and large voids with the diameter of 2.7 Å. The large voids in alpha-selenourea can be connected with its collapse under pressure and the transformation to more dense phase beta.
The reversible transition from phase α compressed in glycerine to phase beta takes place at 0.21 GPa. High-pressure study revealed that the compressibility of phase alphain water is smaller than in glycerine. This result suggests that some water molecules can penetrate into the voids and therefore the crystal initially increases its volume. Apparently, some of the voids are inaccessible for the water molecules and therefore such partly hydrated alpha-selenourea crystals transform to phase β at 0.30 GPa, this pressure is about 0.10 GPa higher than in case when glycerine was used as the hydrostatic fluid. The penetration of H2O into the voids of α- selenourea can be considered as a precursor of the compound hydration
It has established that high pressure destabilizes the structure of α-selenourea and reduces its exceptional high Z’ from 9 to 2. It appears that the large Z’ number in α-selenourea is a consequence of no sharp preference of the molecules to aggregate in one specific way. This feature of the crystal structure can be described as a labile crystal environment of molecules.
Owing to the presence of voids, somewhat smaller than the size of water molecules, alpha-selenourea is differently compressed in water and in liquids consisting of larger molecules.